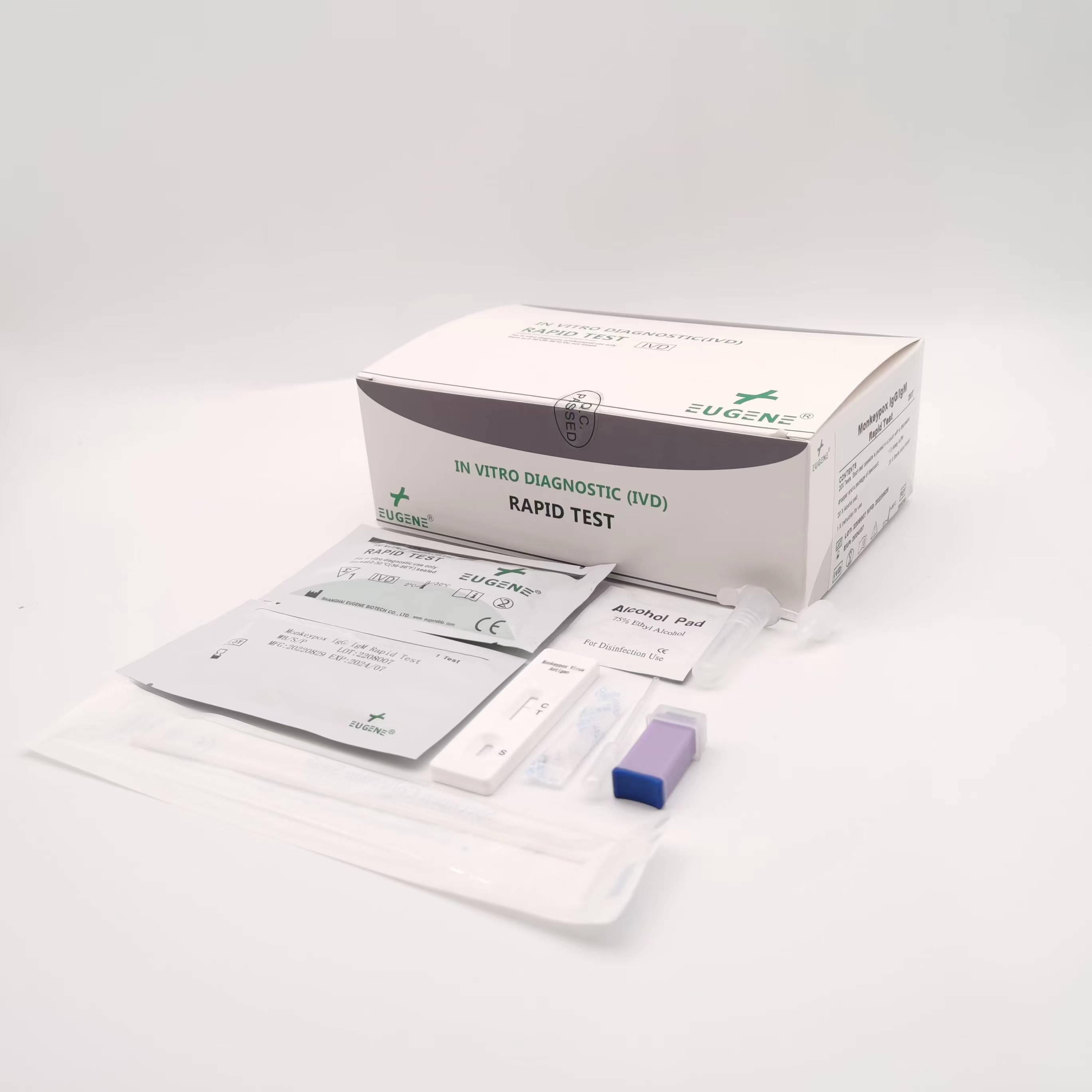
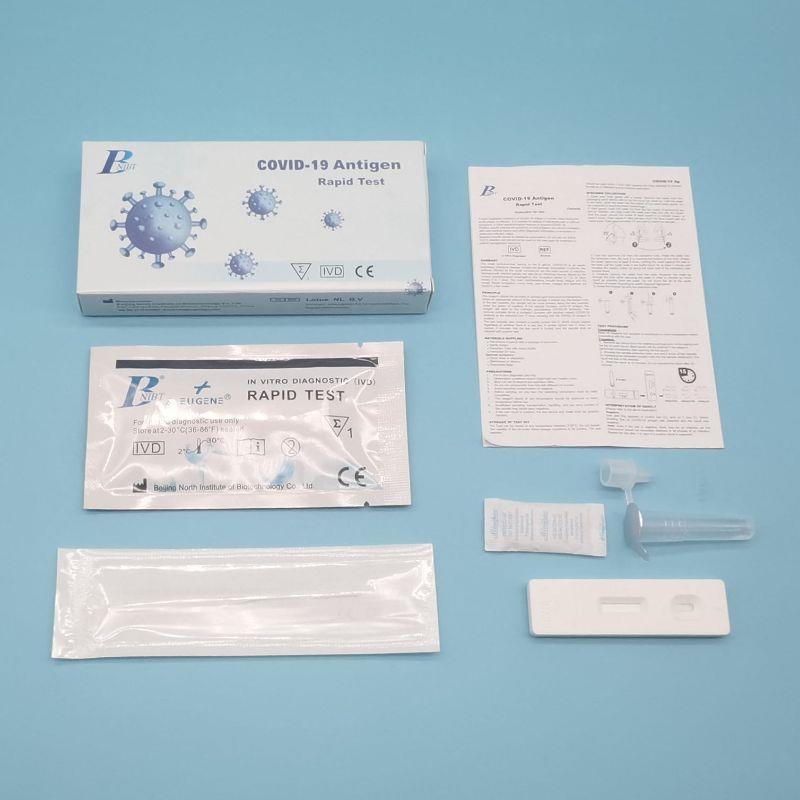
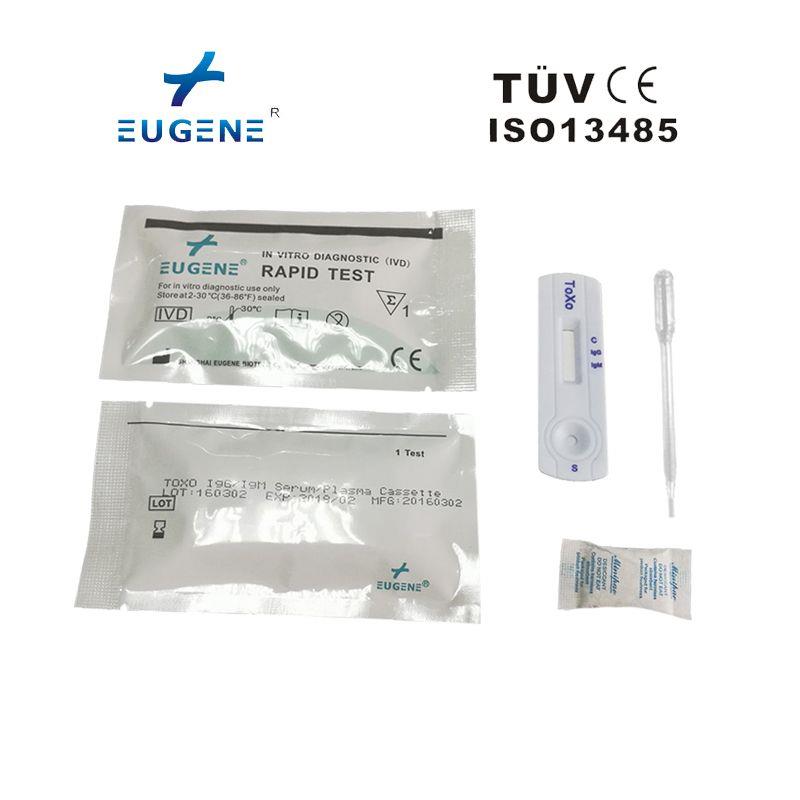
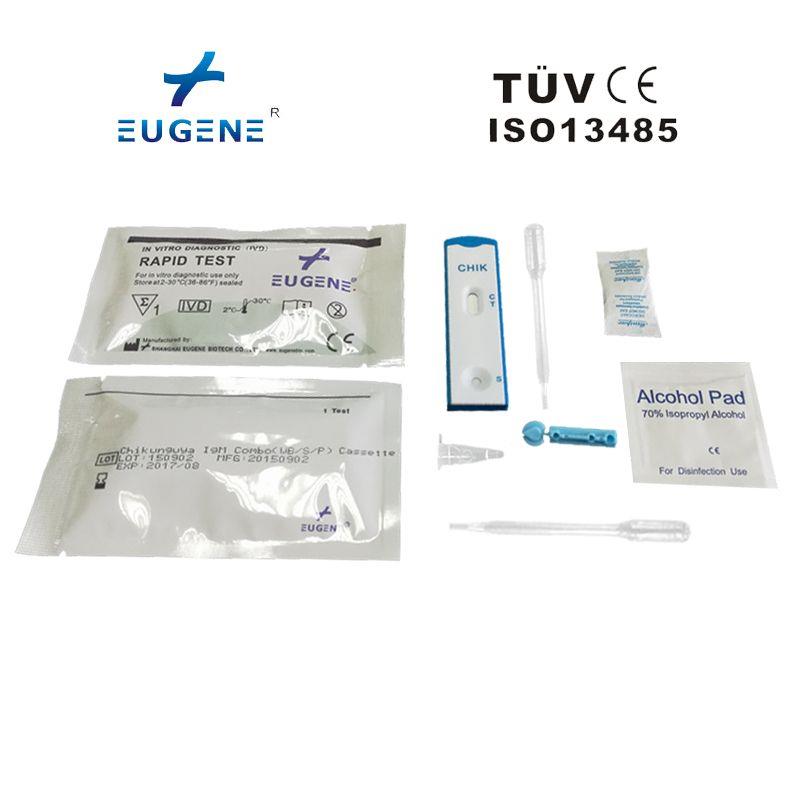
product description
Model: Colloidal Gold Immunoassay Technologies Certification: CE
Dimension:63cm*41cm*29cm Packaging Details: Eugene color pouch and inner box, fully packed in export cartons
Brand Name: Eugene Place of Origin: Shanghai
Warranty: 2 years Color: white
Size: strip/cassette/midstream Finish: 7-15Days
Customer's Logo: Eugene MOQ: 500pcs
Processing Time: 5-10 minutes
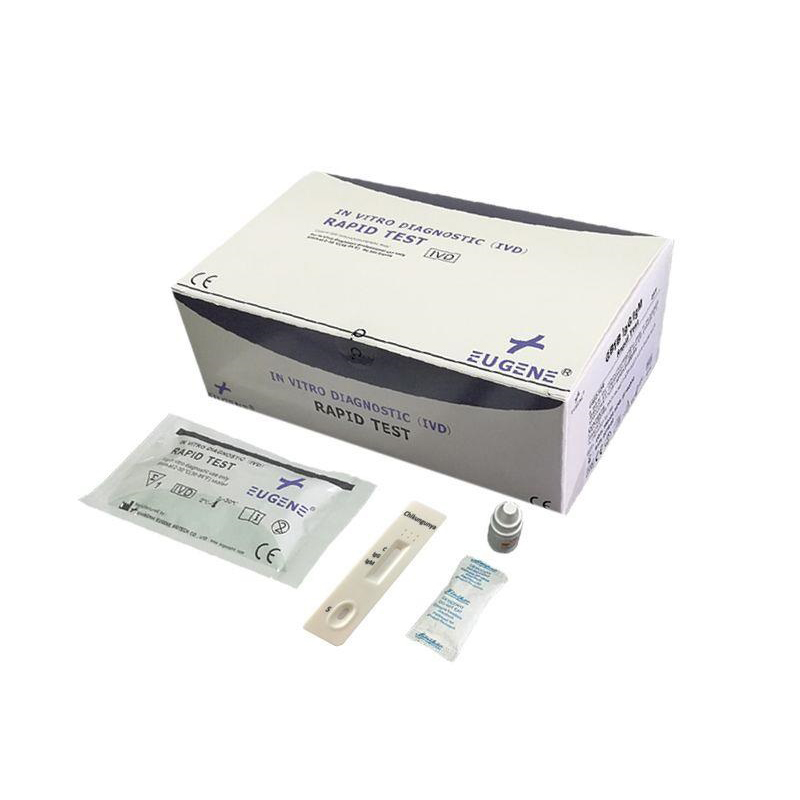
Usage: The EUGENE® Chikungunya IgG/IgM Rapid Test is a lateral flow immumochromatographic assay for the qualitative detection and differentiation of IgG and/or IgM antibodies to Chikungunya (CHIK) in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with Chikungunya. The test is recommended for professional use only. All results must be interpreted together with other clinical information available to the physician.
Quality: sensitivity:IgG:94.30%,IgM:90.30% ; accuracy:IgG:95.60%,IgM:92.50%
Packaing: 25T/kit
SUMMARY
The Chikungunya virus (CHIKV) is an enveloped positive strand RNA virus belong to family Togaviridae with genus Alphavirus, first identified in 1953, CHIk fever is transmitted to humans by the bite of a variety of mosquitoes, including Ae.aegypti, Ae.Albopictus, Aedes afiricanus, Ae.luteocephalus, Ae.furcifer and Ae.Taylori. CHIKV has caused outbreaks in East Africa (Tanzania and Uganda), in Austral Africa (Zimbabwe and South Africa), in West Africa (Senegal and Nigeria), and in central Africa (Central Africa republic and Democratic Republic of the Congo). In Asia, CHIKV outbreaks have been reported in India, Sri Lanka Myanmar, Thailand, Indonesia, the Philippines, Cambodia, Vietnam, Hong Kong and Malaysia, symptoms of sudden onset of fever, chills, headache, nausea, vomiting, joint pain with or without swelling, low back pain ,and rash are very similar to those of dengue. Both diseases are transmitted by the same species of the mosquitoes Aedes aegypti and Ae. Albopictus and mixed outbreak of Chikungunya, with sporadic cases of dengue has been reported in Andhra Pradesh state, India. However, unlike dengue, there is no hemorrhagic or shock syndrome from. Therefore, the ability to distinguish CHIKV infection from dengue virus infection would be extremely beneficial, particularly in areas where dengue virus infection is endemic or epidemic.
The EUGENE® Chikungunya IgG/IgM Rapid Test is an immunochromatographic assay that uses specific antigen-coated colloidal gold to detect the presence of IgG and IgM anti-Chikungunya virus (CHIK) in whole blood or serum specimens. The test is simple and easy to perform by untrained or minimally skilled personnel without burdensome lab equipment requirement. The test results can be visually interpreted within 15 minutes.
STORAGE AND STABILITY
Store the kit in cool and dry places at a temperature between 2-30°C. Do not freeze. The shelf-life of the kit under these storage conditions is 24 months.
SPECIMEN COLLECTION AND PREPARATION
The EUGENE® Chikungunya IgG/IgM Rapid Test can be performed using whole blood (from either venipuncture or fingerstick), serum or plasma.




 Ordinary
Ordinary
 verified
verified