Shenzhen IMDK Medical Technology Co., Ltd.
Shenzhen IMDK Medical Technology Co., Ltd.


favorite supplier

Shenzhen IMDK Medical Technology Co., Ltd.
Shenzhen IMDK Medical Technology Co., Ltd. is headquartered in Shenzhen Guangming Science City, covering 2,000 square meters. The group’s subsidiary in Changsha, Hunan, spans 3,500 square meters, featuring a 1,000+ square meter 100,000-class cleanroom, along with standardized workshops and production lines. The company specializes in the design, R&D, production, and sales of Class II medical devices, offering a wide range of products including medical and home-use mesh nebulizers, fetal Dopplers, pulse oximeters, blood pressure monitors, blood glucose and uric acid monitors, as well as other emergency respiratory support devices.
Many of IMDK’s products have obtained EU CE certification, FDA (510K) clearance, and NMPA (formerly CFDA) certification. The company holds over 100 intellectual property rights, including invention patents, utility model patents, design patents, and software copyrights.
IMDK places strong emphasis on systematic management and quality assurance. It has obtained certifications such as GB/T29490 Enterprise Intellectual Property Management System, ISO 13485 Medical Device Quality Management System, and MDSAP. With a robust quality management system, strict quality control, and reliable after-sales service, the company is committed to ensuring product excellence and safeguarding customers’ health.
Many of IMDK’s products have obtained EU CE certification, FDA (510K) clearance, and NMPA (formerly CFDA) certification. The company holds over 100 intellectual property rights, including invention patents, utility model patents, design patents, and software copyrights.
IMDK places strong emphasis on systematic management and quality assurance. It has obtained certifications such as GB/T29490 Enterprise Intellectual Property Management System, ISO 13485 Medical Device Quality Management System, and MDSAP. With a robust quality management system, strict quality control, and reliable after-sales service, the company is committed to ensuring product excellence and safeguarding customers’ health.
 CE
CE CE
CE CE
CE CE
CE CE
CE CE
CE CE
CE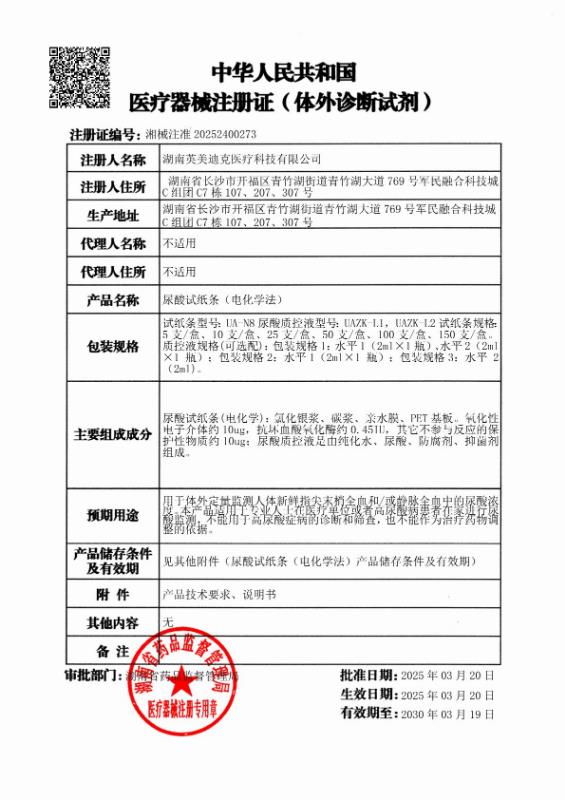 CFDA
CFDA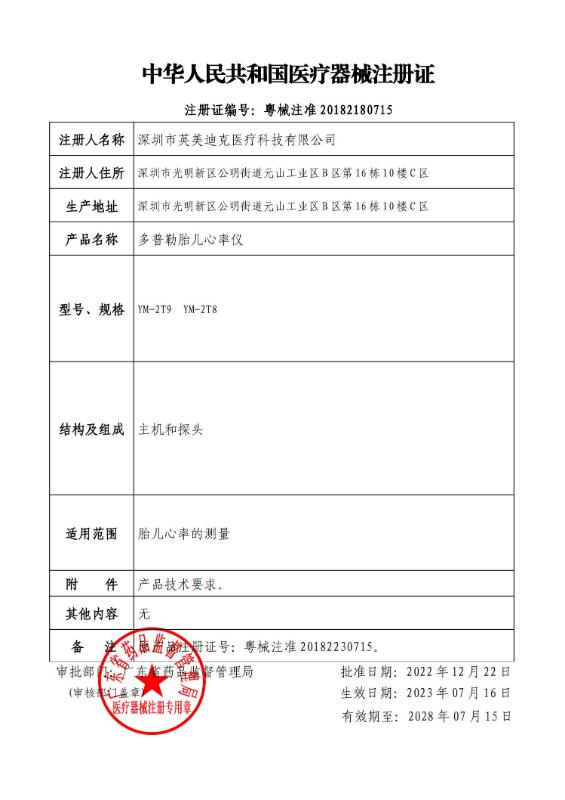 CFDA
CFDA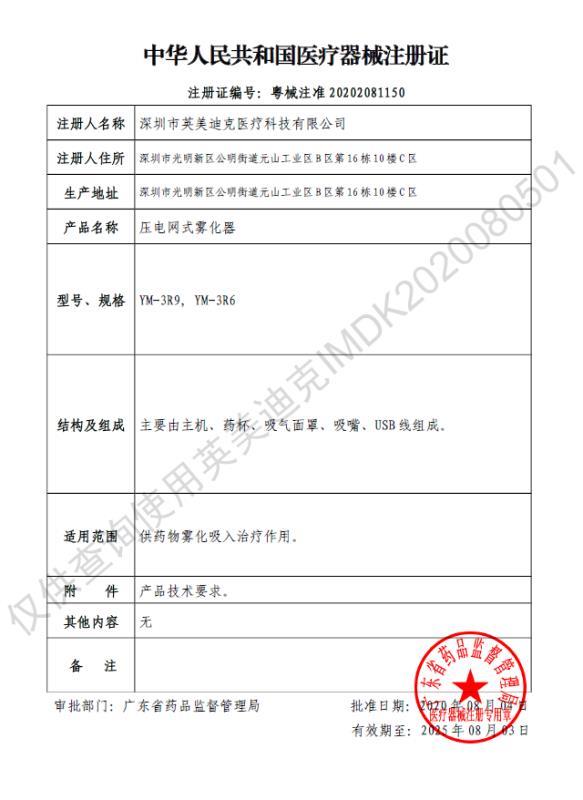 CFDA
CFDA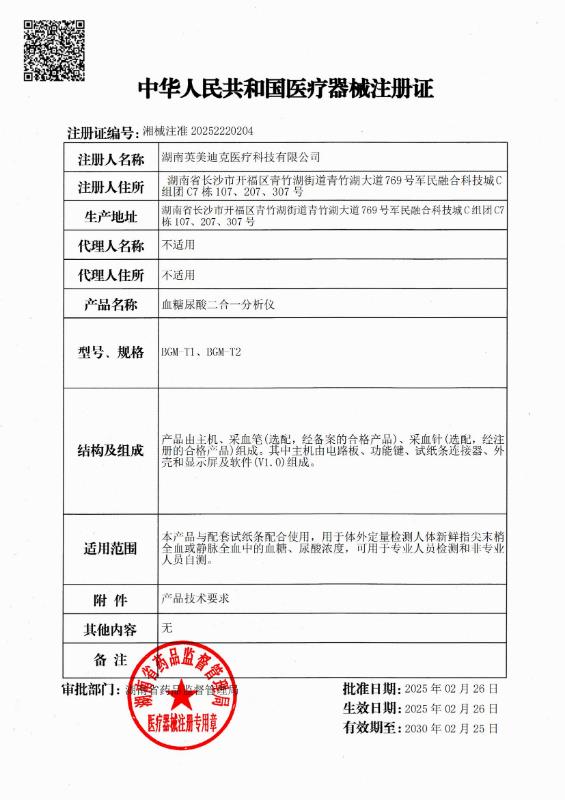 CFDA
CFDA CFDA
CFDA CFDA
CFDA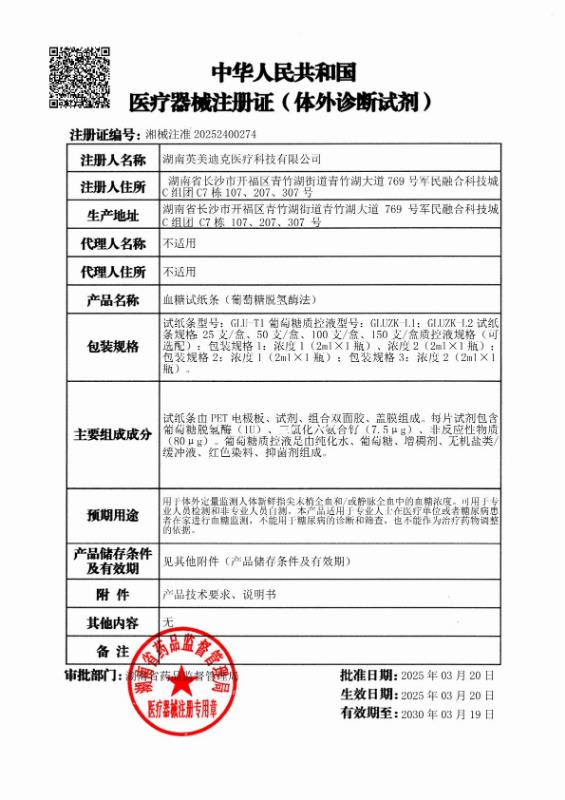 FDA
FDA FDA
FDA FDA
FDA MDSAP
MDSAP FDA
FDA
leave a message
-
 phone :
view details
phone :
view details
-
 address :
9F,Guangming Tianan Cloud Park Building,255 Zhenmei Road,Zhenmei Community,Xinhu Street, Guangming District,Shenzhen, PEOPLE'S REPUBLIC OF CHINA.
address :
9F,Guangming Tianan Cloud Park Building,255 Zhenmei Road,Zhenmei Community,Xinhu Street, Guangming District,Shenzhen, PEOPLE'S REPUBLIC OF CHINA.
-
 postcode :
518107
postcode :
518107
-
 website :
https://imdk.en.alibaba.com/index.html
website :
https://imdk.en.alibaba.com/index.html










 sign in
sign in
 join free
join free


 Copper
Copper
 verified
verified

 verified
verified
 Copper Member
Copper Member
 verified
verified
 Copper Member
Copper Member

