trending topics
market reports
-

Registration Now Open: MEDICAL JAPAN 2026 OSAKA – Western Japan’s Largest Healthcare Trade Show
2026-02-10
-

MEDICAL JAPAN 2025 OSAKA Returns to Showcase Global Innovations
2025-02-17
-

Visit MEDICAL JAPAN 2023 TOKYO and take full advantage of the business opportunities!
2023-09-01
-

US to distribute 400 million free N95 masks at CVS, Walgreens in COVID fight
2022-01-21
-

Ethiopia receives additional 2.2 mln doses of Chinese-donated COVID-19 vaccines
2022-01-21
-
Hong Kong researchers say they develop novel material able to kill COVID-19 virus
2022-01-14
-

10 million more Chinese doses on way for Kenya
2022-01-14
-

Sino-African ties on track for a brighter future
2022-01-07
-
Efforts urged to boost COVID-19 vaccine production capacity in poor countries
2022-01-07
-
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
Rapid progress made in developing vaccine
2020-09-17
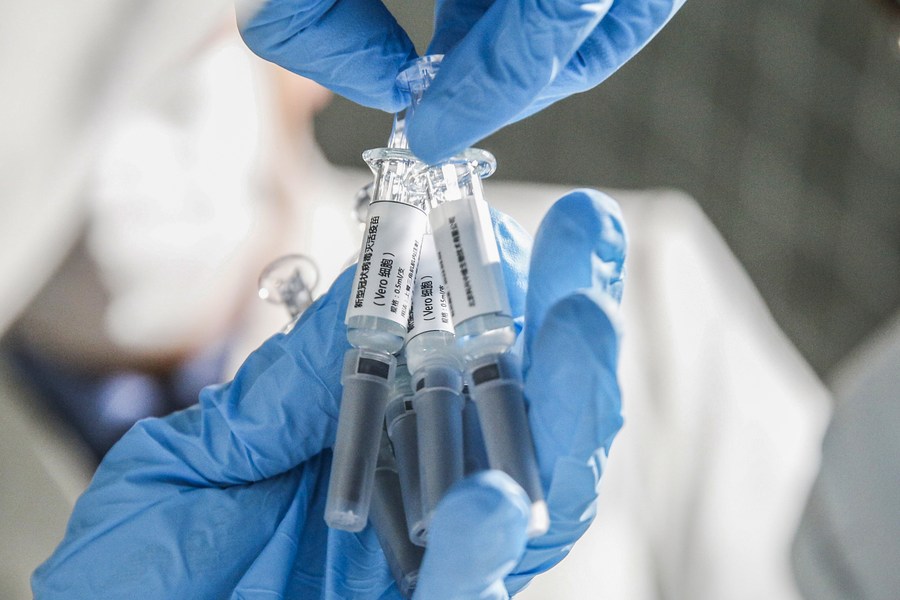
A staff member displays samples of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd, in Beijing, March 16, 2020. [Photo/Xinhua]
Expert says it may be available for public use as early as November
Research and development on COVID-19 vaccines in China is progressing rapidly, and they may be available for public use as early as November, a senior virologist said.
Five of the nine COVID-19 vaccines that have entered phase 3 clinical trials globally are being developed in China, and they have produced satisfying trial results, said Wu Guizhen, chief biosafety expert at the Chinese Center for Disease Control and Prevention. A vaccine usually has to complete three phases of trials before gaining market approval.
None of the people who have received candidate COVID-19 vaccines developed by China for emergency use have exhibited serious side effects or contracted the novel coronavirus, Wu said.
"It is expected that ordinary people can receive COVID-19 vaccination in November or December," she said. "We expect the vaccinations will remain effective for between one and three years."
Faced with a deadly pandemic, countries have been racing against time to research and develop COVID-19 vaccines. A vaccine can normally take more than a decade to develop.
Effective vaccines have been seen as a crucial weapon in containing the pandemic, which has resulted in nearly 30 million cases worldwide. Although it is effectively under control in China, some experts have called for vigilance to guard against new outbreaks in winter.
Three candidate COVID-19 vaccines have been approved by Chinese authorities for emergency use by groups at higher risk of infection, including some medical workers treating COVID-19 patients and people going overseas to work.
Zhou Song, chief legal adviser of China National Biotec Group, said earlier that two vaccines being developed by the company are undergoing phase 3 clinical trials and have been approved for emergency use. Zhou said they have proved to be safe and effective in creating an immune response to the virus.
Gao Fu, director of the China CDC, said at the first Greater Bay Area Vaccine Summit, which was held in Shenzhen, Guangdong province, over the weekend that groups at greater risk should be given priority for immunization with COVID-19 vaccines, including those going to countries seriously affected by the virus for work, front-line health workers and elderly people with chronic diseases.
Wu said that with the arrival of fall and winter, peak seasons for infectious diseases such as flu and COVID-19, vulnerable groups should be able to receive COVID-19 vaccines once they are available as well as flu vaccines.
A technical guide released by the China CDC last week recommended that groups, including medical workers, employees at nursing homes, primary school teachers and students, the elderly and people with chronic diseases should receive flu vaccines.
Senior experts, including Wang Chen, president of the Chinese Academy of Medical Sciences, have also called for measures to be taken to encourage the public to get flu vaccines to prevent the disease in fall and winter to minimize health risks and relieve the burden on medical institutions in case of outbreaks of COVID-19.
Flu vaccinations have already started in some places in China. In Gansu province, for example, 60,000 flu vaccines had been administered as of the end of August. In Beijing, the authorities started buying flu vaccines for fall and winter in August.
(China Daily)



 My Member
My Member Message Center
Message Center