trending topics
market reports
-

Registration Now Open: MEDICAL JAPAN 2026 OSAKA – Western Japan’s Largest Healthcare Trade Show
2026-02-10
-

MEDICAL JAPAN 2025 OSAKA Returns to Showcase Global Innovations
2025-02-17
-

Visit MEDICAL JAPAN 2023 TOKYO and take full advantage of the business opportunities!
2023-09-01
-

US to distribute 400 million free N95 masks at CVS, Walgreens in COVID fight
2022-01-21
-

Ethiopia receives additional 2.2 mln doses of Chinese-donated COVID-19 vaccines
2022-01-21
-
Hong Kong researchers say they develop novel material able to kill COVID-19 virus
2022-01-14
-

10 million more Chinese doses on way for Kenya
2022-01-14
-

Sino-African ties on track for a brighter future
2022-01-07
-
Efforts urged to boost COVID-19 vaccine production capacity in poor countries
2022-01-07
-
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
WHO urges caution about hopes for COVID-19 vaccine
2020-08-04
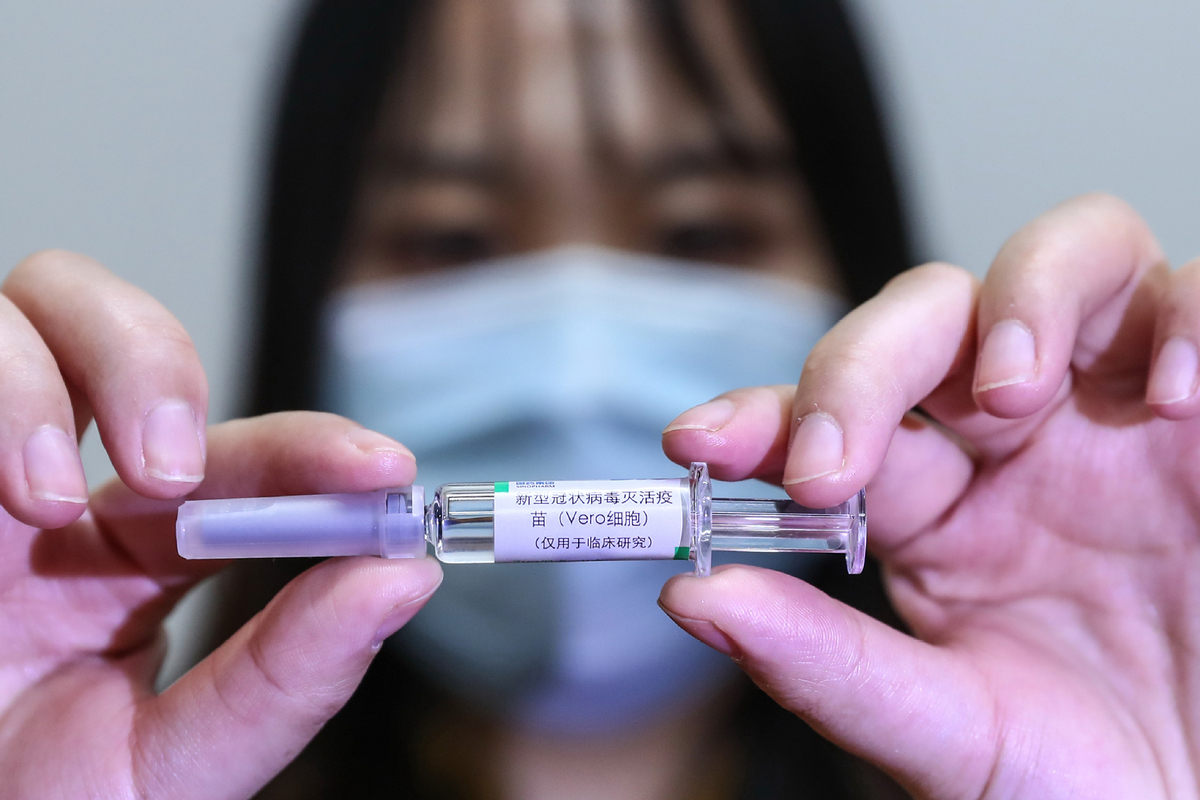
An employee shows a trial vaccine sample at a COVID-19 vaccine production base of Sinopharm in Beijing, April 10, 2020. [Photo/Xinhua]
The World Health Organization, or WHO, warned on Monday that while COVID-19 vaccines undergoing clinical trials may give people hope of protection from infection, it is also possible that they may not work as desired.
There are now 165 COVID-19 vaccine candidates in development, with five already in phase-three clinical trials, according to a WHO report on July 31.
"We all hope to have a number of effective vaccines that can help prevent people from infection," WHO Director-General Tedros Adhanom Ghebreyesus said from Geneva during a virtual news conference.
"However, there is no silver bullet at the moment and there might never be."
Tedros noted that the speed for development of COVID-19 vaccine has reached an unprecedented level. "And there is hope. It doesn't mean that we will have the vaccine," he said.
He said there are concerns that "we may not have a vaccine that may work, or its protection could be for just a few months, not more", adding that until the clinical trials are finished, people will not know if they have a vaccine that can protect effectively and in the long term.
In the mean time, Tedros asked people to focus on measures that are to hand that can help suppress and control the novel coronavirus, such as testing, isolating and treating patients, tracing and quarantining their contacts, informing and empowering communities and strengthening healthcare systems and national unity and global solidarity.
Anthony Fauci, director of the United States' National Institute of Allergy and Infectious Diseases, told lawmakers at a hearing on Friday that a COVID-19 vaccine could be ready for distribution by the end of the year, and distributed to US citizens in 2021.
"From everything we've seen now, in the animal data, as well as the human data, we feel cautiously optimistic that we will have a vaccine by the end of this year and as we go into 2021," he said.
The European Union and the United Kingdom are also racing to secure possible vaccines for their citizens.
The European Commission announced on Friday that on behalf of EU member states, it has made a reservation for 300 million doses of a vaccine being developed by Sanofi and GSK.
"The European Commission does all in its power to ensure that Europeans have rapid access to a vaccine that is safe and protects them from coronavirus," Commission President Ursula von der Leyen said in a news release.
The UK has signed a deal with GSK and Sanofi for 60 million doses of a potential COVID-19 vaccine. It is the fourth deal of this kind the government has signed in recent months, for a total of 250 million doses so far, the BBC reported.
Russia's health minister, Mikhail Murashko, said on Saturday that the country will start a mass vaccination campaign against COVID-19 from October.
The vaccination will be free of charge and doctors and teachers would be the first to be vaccinated, Turkey's Anadolu Agency reported.
(China Daily)



 My Member
My Member Message Center
Message Center