

FOB Price : Get a Price/Quote
Min.Order : 2000 Box(es)
Certification : CFDA,CE,FDA
Brand Name : Neutral or OEM
Payment Terms : L/C,T/T
brand name : Neutral or OEM
certification : CFDA,CE,FDA
min.order : 2000 Box(es)
warranty : 3 years
payment terms : L/C,T/T
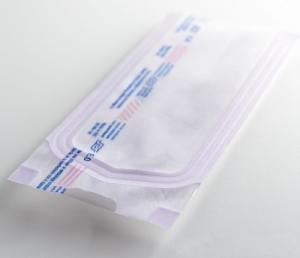
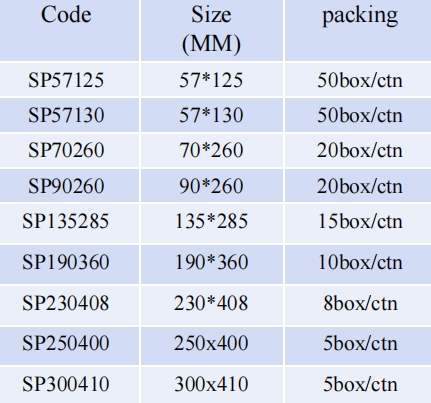
Packaging : 57mm*125mm:50box/ctn;57mm*130mm:50box/ctn;70mm*260mm:20box/ctn;etc.
Specification : 57mm*125mm;57mm*130mm;70mm*260mm;90mm*260mm,etc.
place of origin : Anhui





 Ordinary
Ordinary
 verified
verified
Business Type Service
Country / Region Anhui,China
Main Products Disposable medical consumables, Sterilization & Disinfection Consumables
Main Markets India,Saudi Arabia,Dubai,Australia,Brazil,Egypt

Brand : Neutral or OEM
Min.Order : 400,000 Vial(s)

Brand : Neutral or OEM
Min.Order : 2000 Kg(s)

Brand : Neutral or OEM
Min.Order : 200,000 Piece(s)

Brand : GUTAIKE or Customized logo
Min.Order : 200,000 Piece(s)

Brand : Neutral or OEM
Min.Order : 2000 Kg(s)
brand name : Neutral or OEM
certification :
fob price :
min.order : 2000 Box(es)
warranty : 3 years
payment terms : L/C,T/T
Packaing : 57mm*125mm:50box/ctn;57mm*130mm:50box/ctn;70mm*260mm:20box/ctn;etc.
Specification : 57mm*125mm;57mm*130mm;70mm*260mm;90mm*260mm,etc.
Trademark : Neutral or OEM
Production Capacity :
place of origin : Anhui
Manag Certifica : CFDA,CE,FDA

Chemical Indicator Strips Indicator Card Medical Supply

Medical Dialysis Paper Blister Paper Blister Paper for Composite with Film

Self Sealing Sterilization Pouch with Medical Grade Paper and CPP/Pet Film

Medical Packing Film CPP/Pet PE/Pet Composite White Green Blue Purple Film

Printing Adhesive Coated Medical Paper Roll for Sterile Reel Paper-Plastic Pouch for Syringe Set Infusion