








FOB Price : Get a Price/Quote
Min.Order : 100,000 Piece(s)
Certification : CE,FDA,FDA510K
Brand Name : N/A, OEM available
Payment Terms : T/T
brand name : N/A, OEM available
certification : CE,FDA,FDA510K
min.order : 100,000 Piece(s)
warranty : 5 years (sterile package)
payment terms : T/T
Packaging : Blister, Box, Carton
Specification : 0.05ml-10ml
place of origin : Shanghai





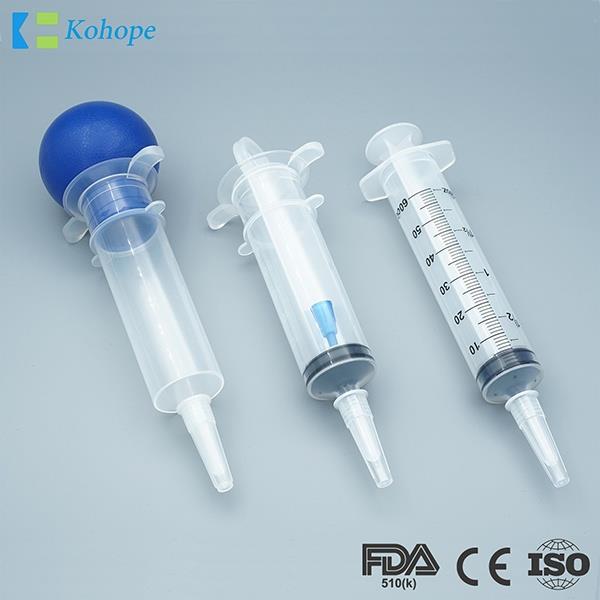
 Ordinary
Ordinary
 verified
verified
Business Type Manufacturer
Country / Region Shanghai,China
Main Products Oral & Dental Equipment, Injection Puncture, Basic Surgical Instrument, Disposable medical consumables, Experimental Consumables, Plastic Surgery Consumables, Stomatology Consumables, Disposables & Consumables, Sterilisation
Main Markets
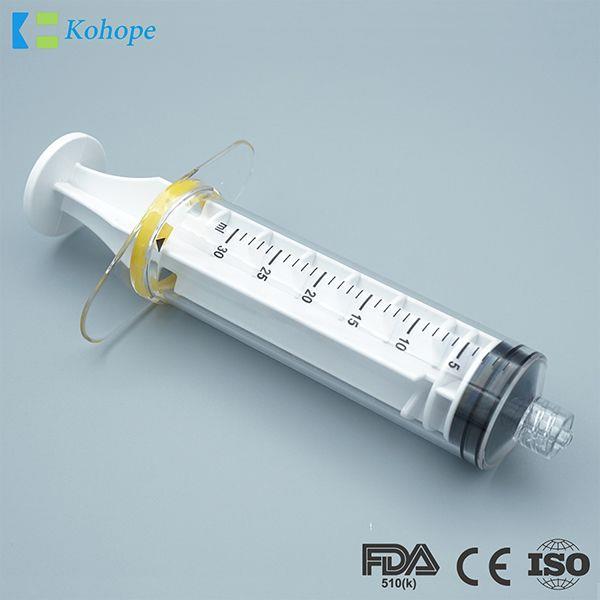
Brand : N/A, OEM available
Min.Order : 10,000 Piece(s)
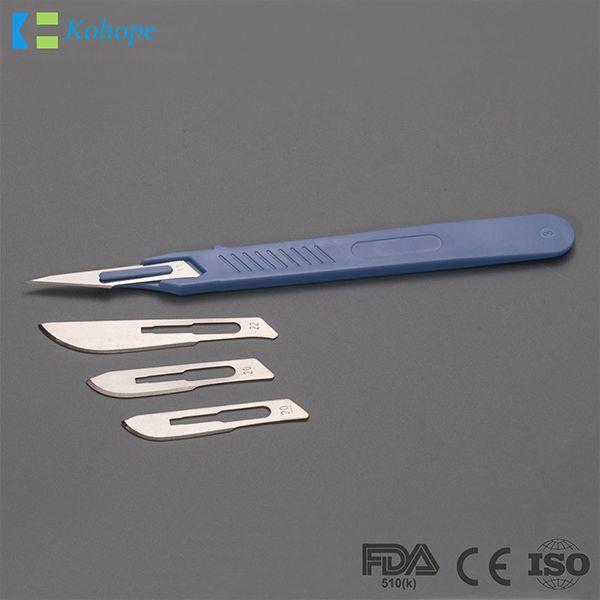
Brand : N/A, OEM available
Min.Order : 100,000 Piece(s)

Brand : N/A, OEM available
Min.Order : 20,000 Piece(s)
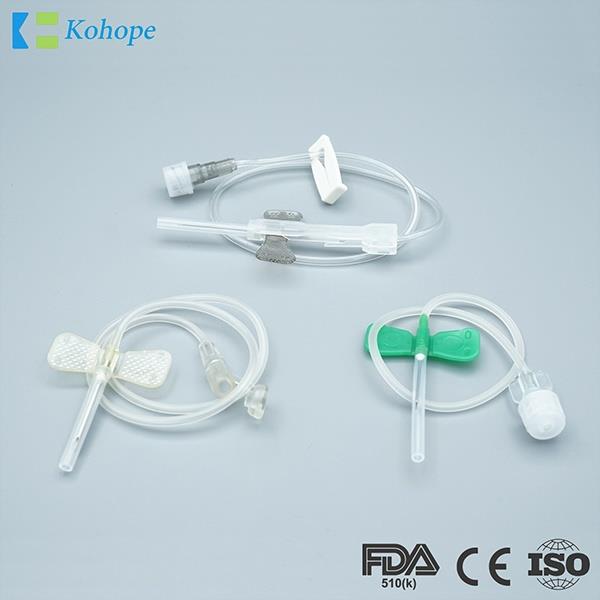
Brand : N/A, OEM available
Min.Order : 100,000 Piece(s)

Brand : N/A, OEM available
Min.Order : 100,000 Vial(s)
brand name : N/A, OEM available
certification :
fob price :
min.order : 100,000 Piece(s)
warranty : 5 years (sterile package)
payment terms : T/T
Packaing : Blister, Box, Carton
Specification : 0.05ml-10ml
Trademark : N/A, OEM available
Production Capacity :
place of origin : Shanghai
Manag Certifica : CE,FDA,FDA510K
