



FOB Price : Get a Price/Quote
Min.Order : 1000 Piece(s)
Certification : CFDA,GMP,ISO13485,CE,FDA,Others
Brand Name : Diagnos
Payment Terms : T/T,WesternUnion,EXW
brand name : Diagnos
certification : CFDA,GMP,ISO13485,CE,FDA,Others
min.order : 1000 Piece(s)
warranty : 1 year
payment terms : T/T,WesternUnion,EXW
Packaging : 2000pcs/carton
Specification : 40pcs/box,25pcs/box
place of origin : unknown
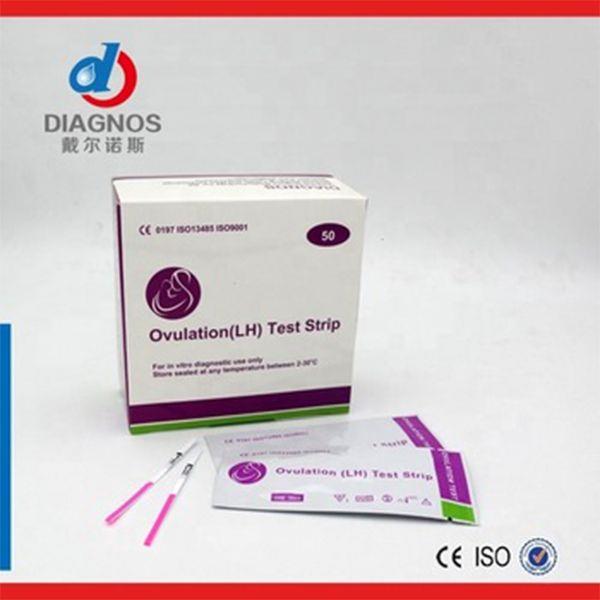
The fresh urine samples should be collected in a clean ,dry container such that same-day testing may be preformed . Urine specimens may be refrigerated at 2-8℃ for 48 hours,or frozen at -20℃ for assaying at a later date .Specimens that have been refrigarated or frozen must be equilibrated to room temperature before testing.Urine samples exhibiting visible precipitates should be filtered,centrifuged or allowed to settle so that clear aliquots can be obtained for testing.
RODUCTION1. Review “ Specimen collection ” instructions. Test device, patient’s samples, and controls should be brought to room temperature (20-30 ℃) prior to testing. Do not open pouches until ready to perform the assay.
2. Remove the test device from its protective pouch (bring the device to room temperature before open the pouch to avoid condensation of moisture on the membrane ). Label the device with patient or control number.
3. Immerse the strip into the sample with the arrow end pointing towards the sample. Do not immerse past the MAX (maximum) line. Take the casstte out after 10 seconds and lay the casstte flat on a clean, dry, nonabsorbent surface.
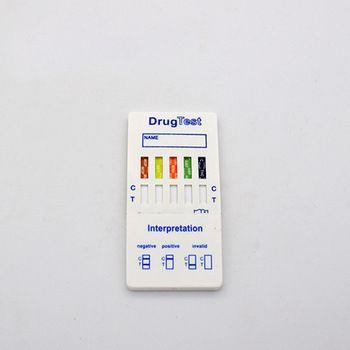
4. Read the tests result at five minutes.
Negative:Two pink-colored bands appear,one in the control region and one in the test region. A negative result indicates free drug is absent from urine or at concentrations lower than the detection cut-off level of the test.
Positive:One pink-colored band appears in the control region with no apparent band appering in the test region. A positive result indicates free drug is present in urine at concentrations at or exceeding the detection cut-off level of the test.
Invalid:No band appears in the control region,or a pink-colored band appears in the test region only. An invalid test result may be due to improper assay procedures or damage to the device. If resultes are invalid, the assay is inconclusive and the specimen should be re-tested using a new test device.
PRECAUTION
1. For in vitro diagnostic use only.
2. Do not use test kit beyond expiry date.
3. The test device should not be reused.



 Ordinary
Ordinary
 verified
verified
Business Type Service
Country / Region Jiangsu,China
Main Products In vitro Diagnosis Reagents
Main Markets Africa,Southeast Asia,Eastern Europe

Brand : Diagnos /OEM
Min.Order : 1000 Piece(s)

Brand : DIAGNOS
Min.Order : 2000 Piece(s)

Brand : DIAGNOS
Min.Order : 5000 Piece(s)

Brand : DIAGNOS
Min.Order : 5000 Piece(s)

Brand : DIAGNOS
Min.Order : 2000 Piece(s)
brand name : Diagnos
certification :
fob price :
min.order : 1000 Piece(s)
warranty : 1 year
payment terms : T/T,WesternUnion,EXW
Packaing : 2000pcs/carton
Specification : 40pcs/box,25pcs/box
Trademark :
Production Capacity :
place of origin : unknown
Manag Certifica : CFDA,GMP,ISO13485,CE,FDA,Others
The fresh urine samples should be collected in a clean ,dry container such that same-day testing may be preformed . Urine specimens may be refrigerated at 2-8℃ for 48 hours,or frozen at -20℃ for assaying at a later date .Specimens that have been refrigarated or frozen must be equilibrated to room temperature before testing.Urine samples exhibiting visible precipitates should be filtered,centrifuged or allowed to settle so that clear aliquots can be obtained for testing.
RODUCTION1. Review “ Specimen collection ” instructions. Test device, patient’s samples, and controls should be brought to room temperature (20-30 ℃) prior to testing. Do not open pouches until ready to perform the assay.
2. Remove the test device from its protective pouch (bring the device to room temperature before open the pouch to avoid condensation of moisture on the membrane ). Label the device with patient or control number.
3. Immerse the strip into the sample with the arrow end pointing towards the sample. Do not immerse past the MAX (maximum) line. Take the casstte out after 10 seconds and lay the casstte flat on a clean, dry, nonabsorbent surface.
4. Read the tests result at five minutes.
Negative:Two pink-colored bands appear,one in the control region and one in the test region. A negative result indicates free drug is absent from urine or at concentrations lower than the detection cut-off level of the test.
Positive:One pink-colored band appears in the control region with no apparent band appering in the test region. A positive result indicates free drug is present in urine at concentrations at or exceeding the detection cut-off level of the test.
Invalid:No band appears in the control region,or a pink-colored band appears in the test region only. An invalid test result may be due to improper assay procedures or damage to the device. If resultes are invalid, the assay is inconclusive and the specimen should be re-tested using a new test device.
PRECAUTION
1. For in vitro diagnostic use only.
2. Do not use test kit beyond expiry date.
3. The test device should not be reused.

