

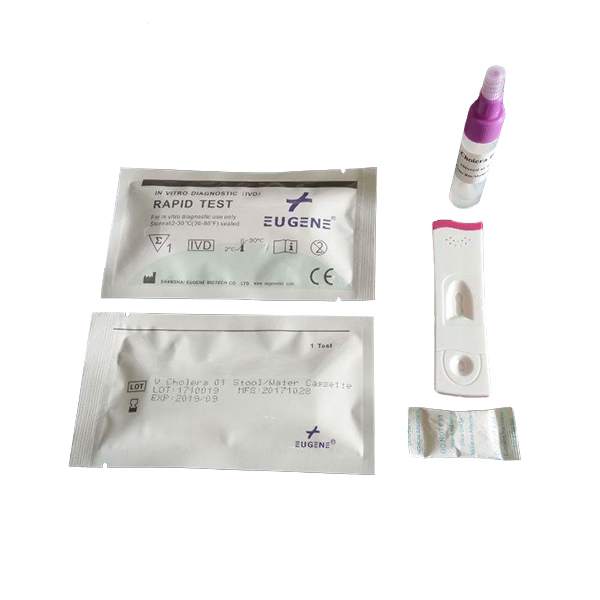
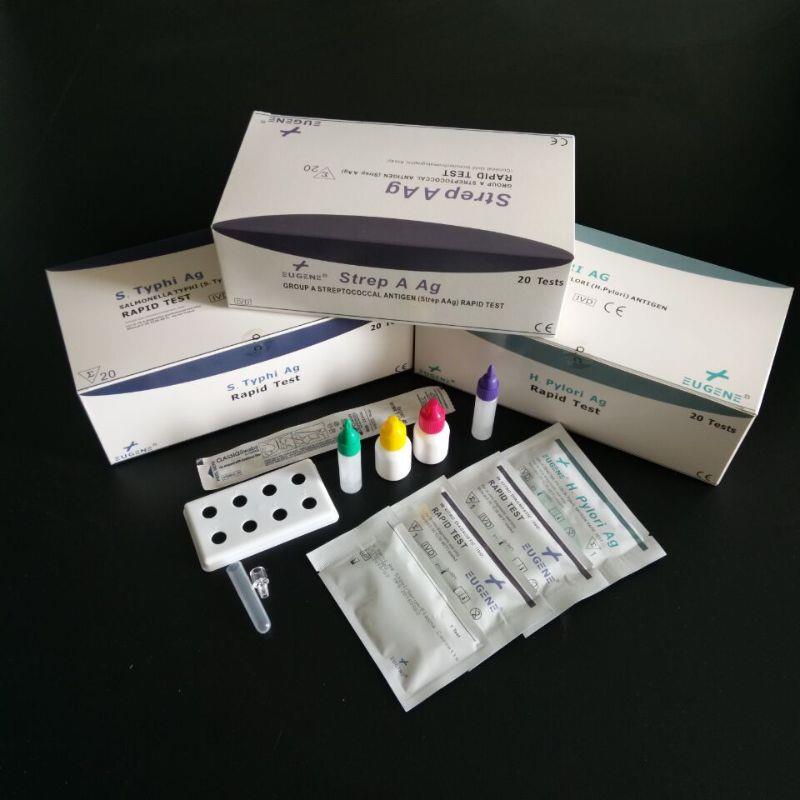
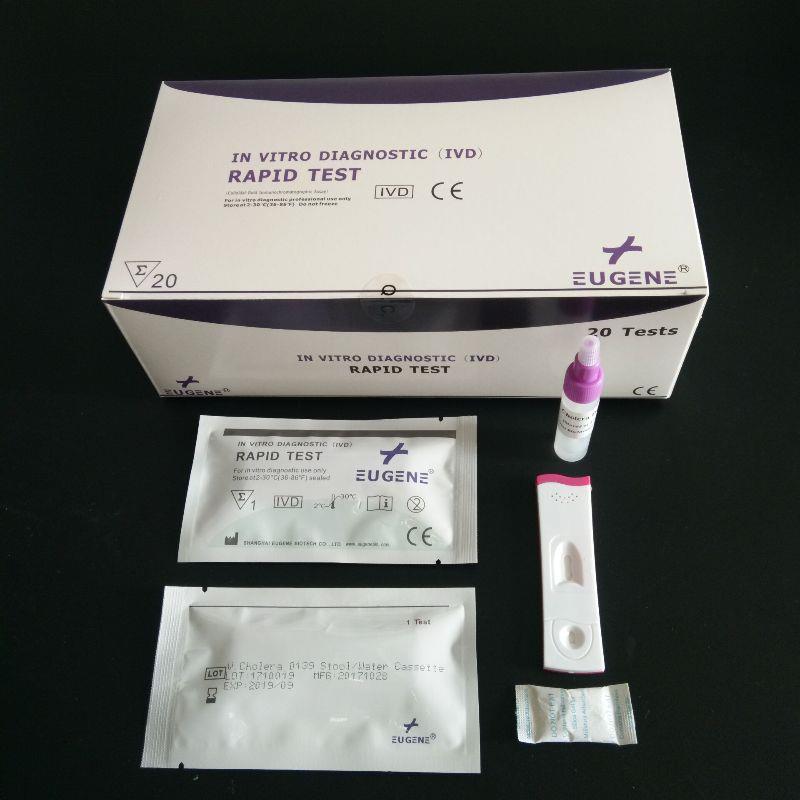
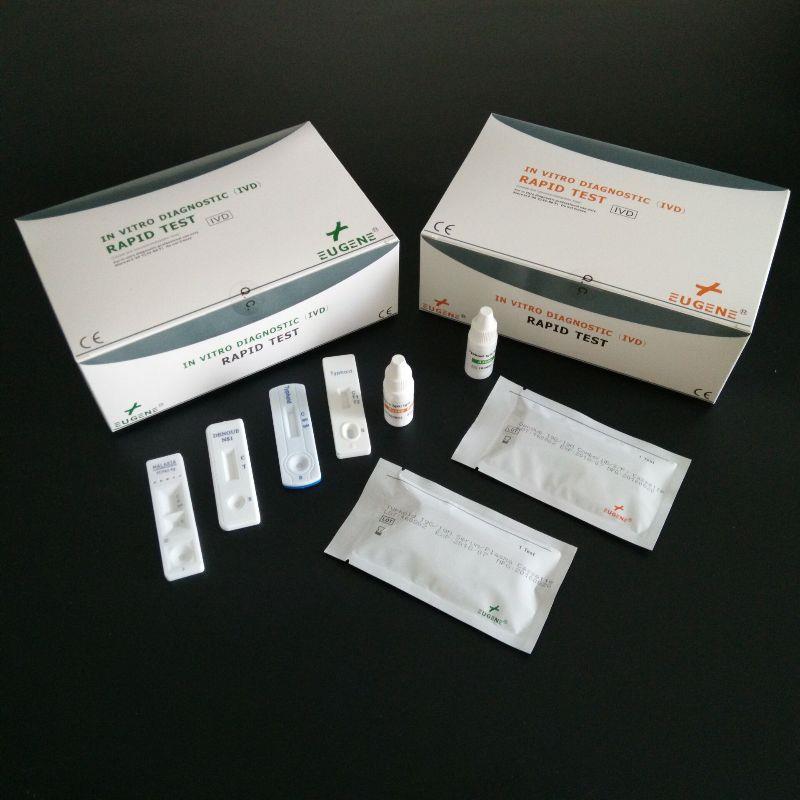
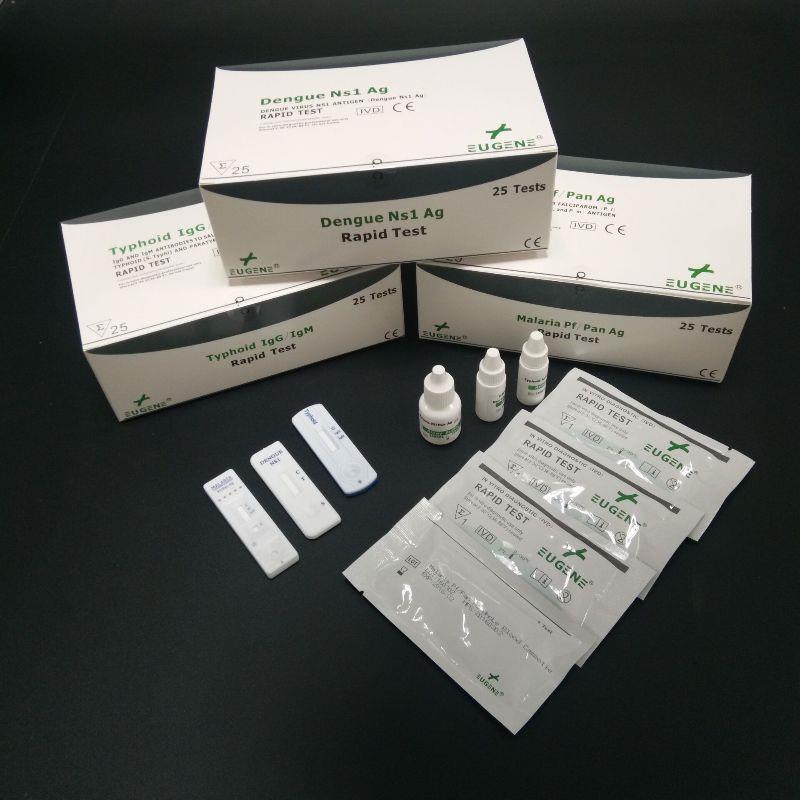
FOB Price : Get a Price/Quote
Min.Order : 1 Set(s)
Certification : CFDA,GMP,ISO13485,CE,FDA
Brand Name : Eugene
Payment Terms : L/C,T/T,WesternUnion
brand name : Eugene
certification : CFDA,GMP,ISO13485,CE,FDA
min.order : 1 Set(s)
warranty :
payment terms : L/C,T/T,WesternUnion
Packaging :
Specification :
place of origin : China
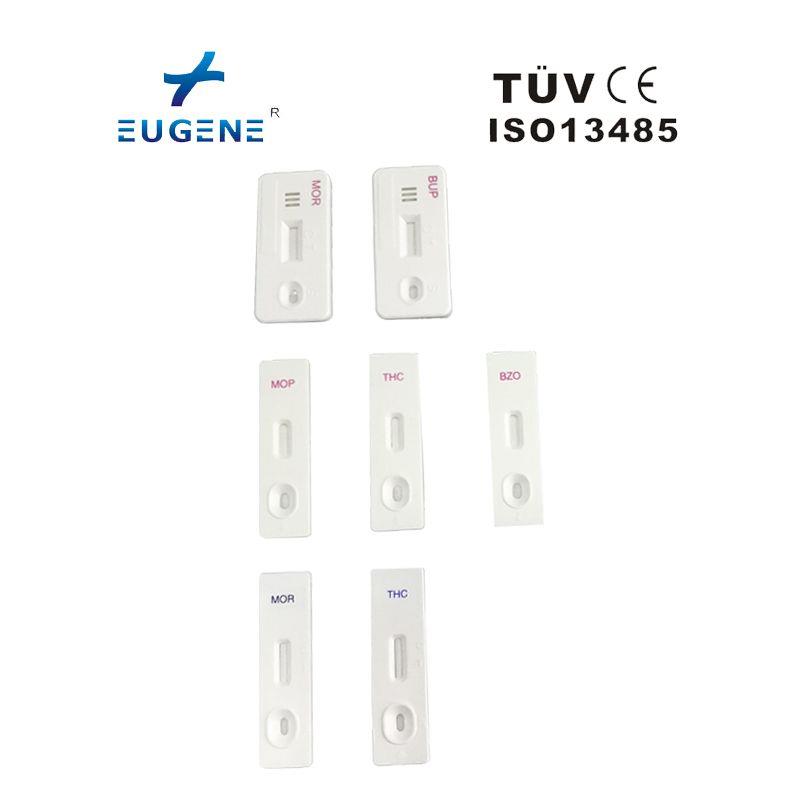

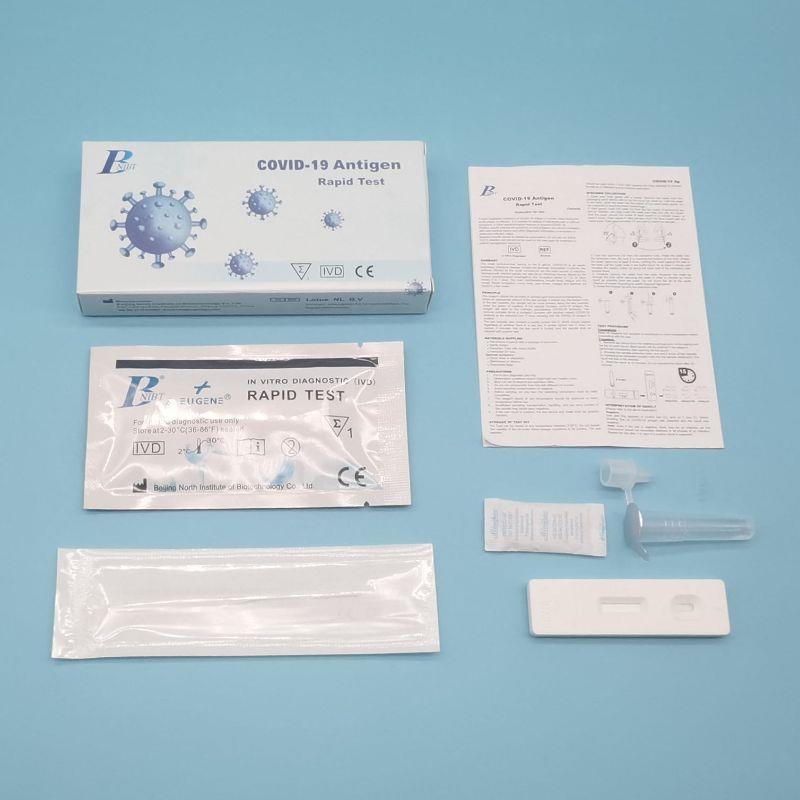

 Ordinary
Ordinary
 verified
verified
Business Type Manufacturer
Country / Region Shanghai,China
Main Products In vitro Diagnosis Reagents, Diagnostic Tests
Main Markets Europe, Asia, Africa, South America, North America
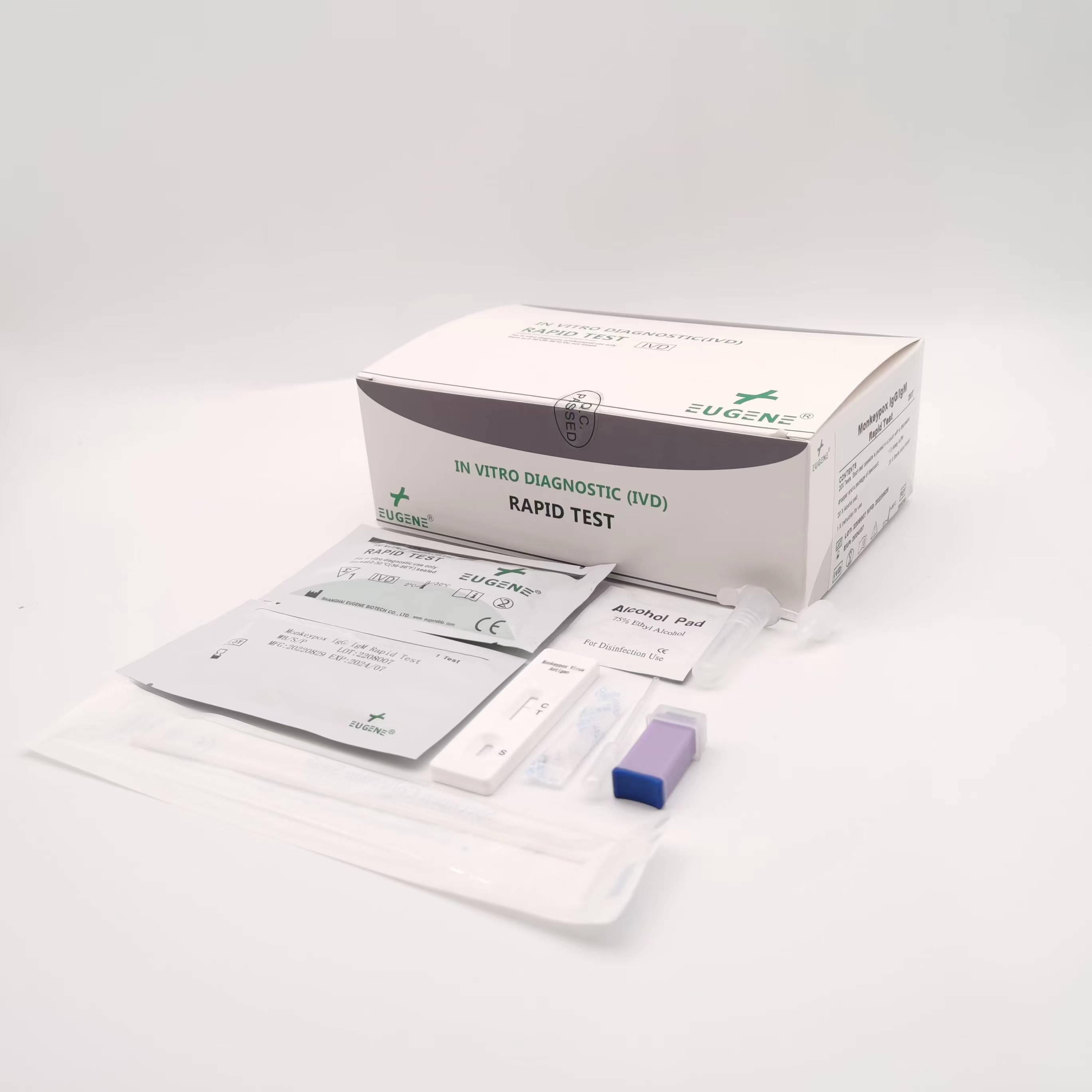
Brand : EUGENE
Min.Order : 500 Piece(s)

Brand : BNIBT
Min.Order : 500 Piece(s)
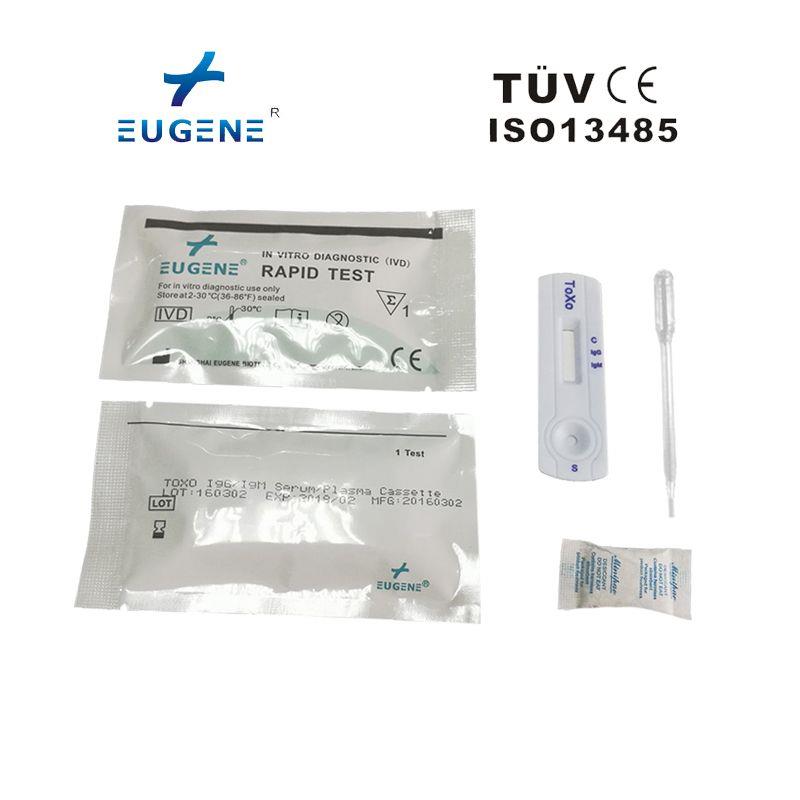
Brand : Eugene
Min.Order : 1 Set(s)

Brand : Eugene
Min.Order : 1 Set(s)

Brand : Eugene
Min.Order : 1 Set(s)
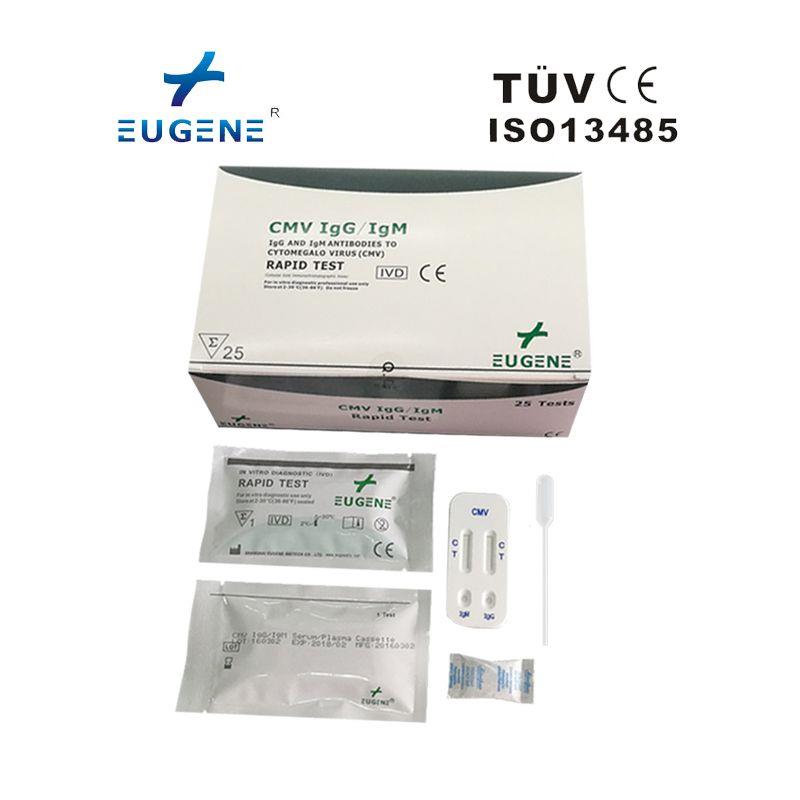
brand name : Eugene
certification :
fob price :
min.order : 1 Set(s)
warranty :
payment terms : L/C,T/T,WesternUnion
Packaing :
Specification :
Trademark : Eugene
Production Capacity :
place of origin : China
Manag Certifica : CFDA,GMP,ISO13485,CE,FDA